

REASSURE Phase 3 Topline Data Conference Call January 30, 2024 EXHIBIT 99.2

This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements regarding the development, therapeutic and market potential of sulopenem, the Company’s ability to address the deficiencies set out in the complete response letter received in July 2021, the expected timing of resubmission of the NDA, the expected timing of review by the FDA, and the Company’s strategic process to sell, license or otherwise dispose of its rights to sulopenem. In some cases, forward-looking statements can be identified by words such as “may,” “believes,” “intends,” “seeks,” “anticipates,” “plans,” “estimates,” “expects,” “should,” “assumes,” “continues,” “could,” “would,” “will,” “future,” “potential” or the negative of these or similar terms and phrases. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include all matters that are not historical facts. Actual future results may be materially different from what is expected due to factors largely outside the Company’s control, including uncertainties inherent in the conduct of clinical and non-clinical development, changes in regulatory requirements or decisions of regulatory authorities, the timing or likelihood of regulatory filings and approvals, including the potential resubmission of the NDA for oral sulopenem, changes in public policy or legislation, commercialization plans and timelines, if oral sulopenem is approved, the actions of third-party clinical research organizations, suppliers and manufacturers, the accuracy of Iterum’s expectations regarding how far into the future Iterum’s cash on hand will fund Iterum’s ongoing operations, Iterum’s ability to maintain its listing on the Nasdaq Capital Market, risks and uncertainties concerning the outcome, impact, effects and results of the Company’s pursuit of strategic alternatives, including the terms, timing, structure, value, benefits and costs of any strategic process and the Company’s ability to complete one at all and other factors discussed under the caption “Risk Factors” in its Quarterly Report on Form 10-Q filed with the SEC on November 14, 2023, and other documents filed with the SEC from time to time. Forward-looking statements contained herein represent the Company’s beliefs and assumptions only as of January 30, 2024. Except as required by law, neither we, nor the Company, assume any obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not been independently verified, and neither we nor the Company make any representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its own internal research is reliable, such research has not been verified by any independent source. Forward-looking Statements & Disclaimer
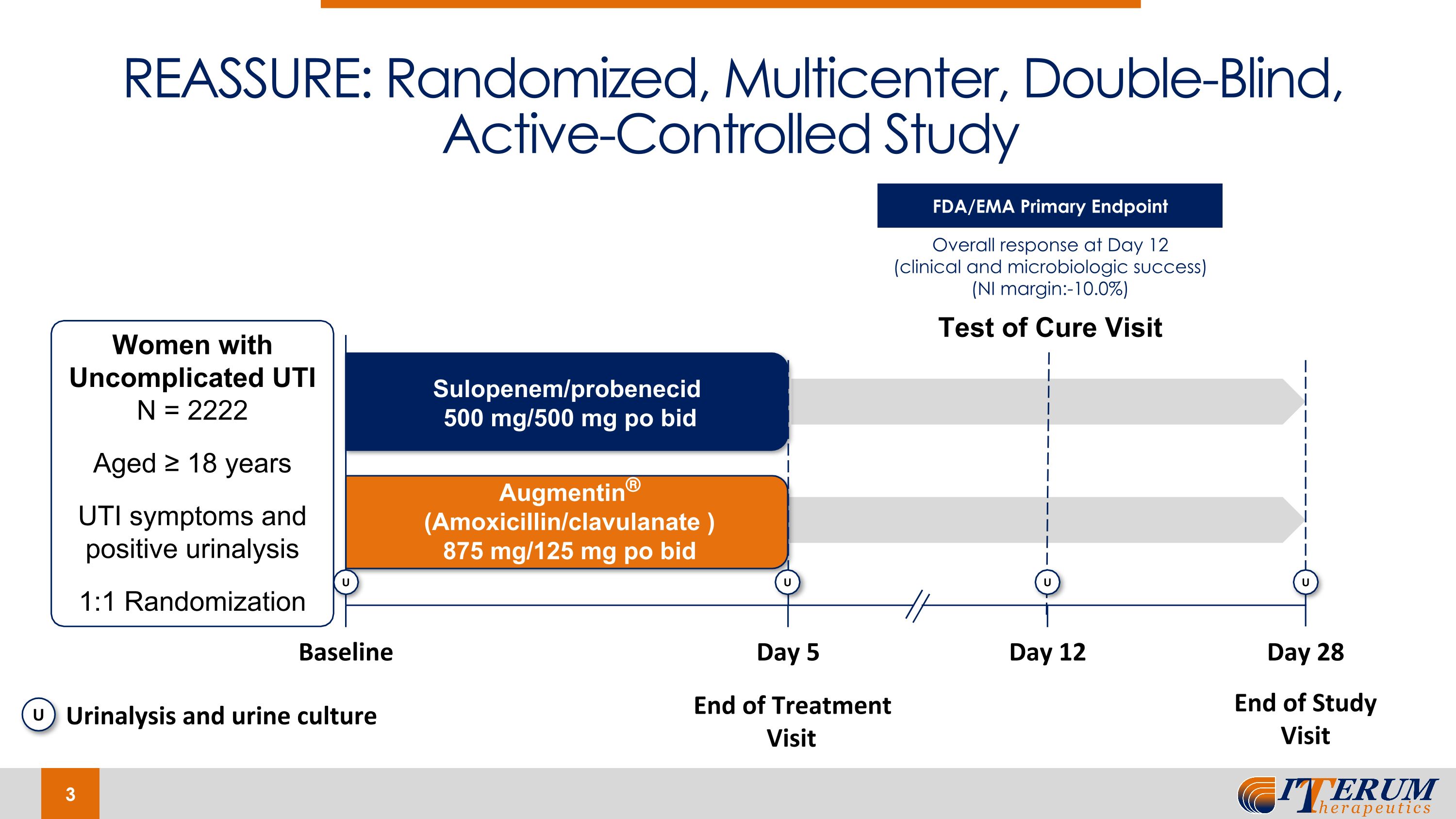
REASSURE: Randomized, Multicenter, Double-Blind, Active-Controlled Study Test of Cure Visit Baseline Day 5 Day 12 Day 28 End of Treatment Visit End of Study Visit Augmentin® (Amoxicillin/clavulanate ) 875 mg/125 mg po bid Sulopenem/probenecid 500 mg/500 mg po bid Women with Uncomplicated UTI N = 2222 Aged ≥ 18 years UTI symptoms and positive urinalysis 1:1 Randomization U U U U U Urinalysis and urine culture FDA/EMA Primary Endpoint Overall response at Day 12 (clinical and microbiologic success) (NI margin:-10.0%)
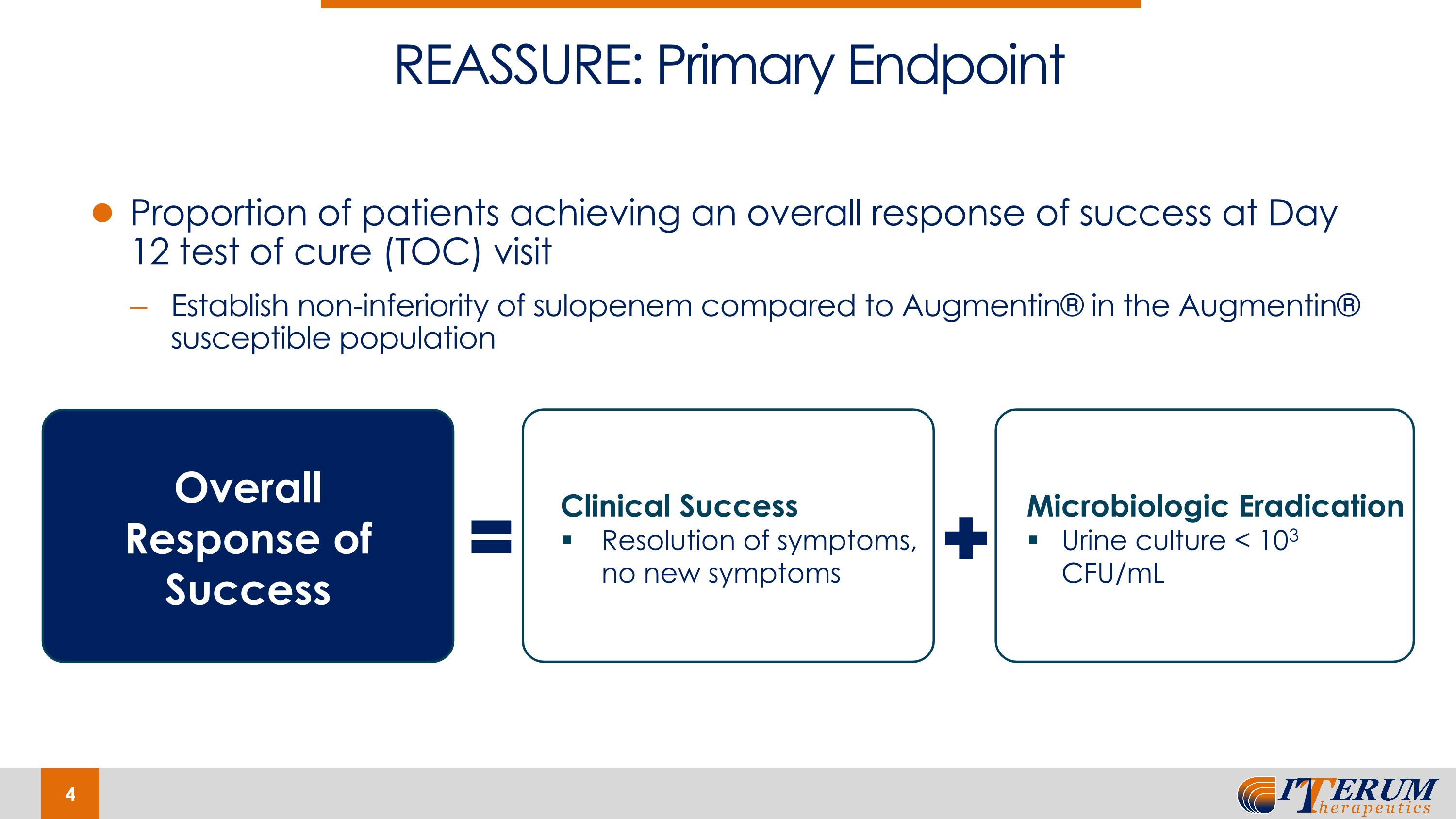
Proportion of patients achieving an overall response of success at Day 12 test of cure (TOC) visit Establish non-inferiority of sulopenem compared to Augmentin® in the Augmentin® susceptible population REASSURE: Primary Endpoint Microbiologic Eradication Urine culture < 103 CFU/mL Overall Response of Success Clinical Success Resolution of symptoms, no new symptoms
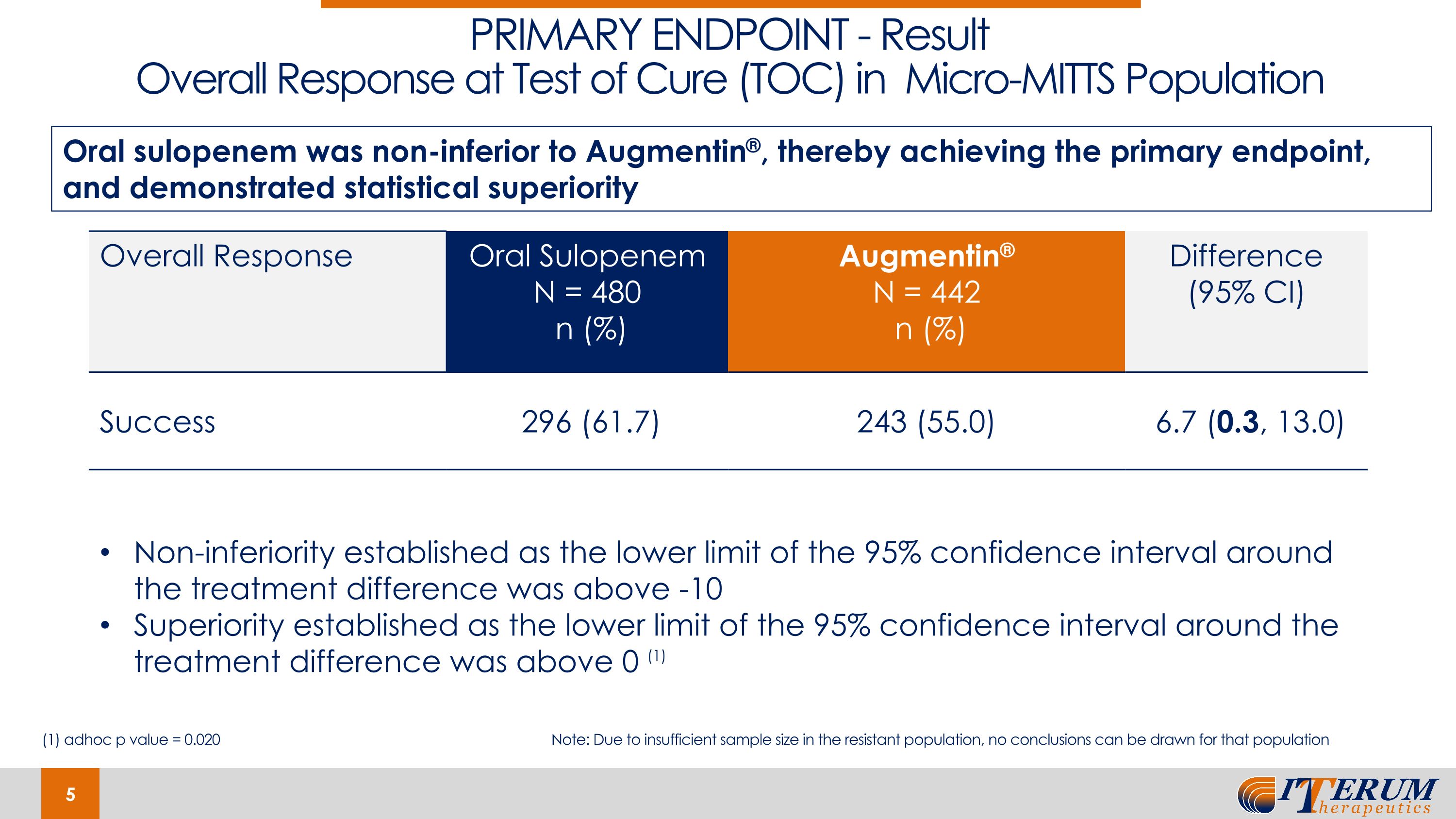
PRIMARY ENDPOINT - Result Overall Response at Test of Cure (TOC) in Micro-MITTS Population Overall Response Oral Sulopenem N = 480 n (%) Augmentin® N = 442 n (%) Difference (95% CI) Success 296 (61.7) 243 (55.0) 6.7 (0.3, 13.0) Oral sulopenem was non-inferior to Augmentin®, thereby achieving the primary endpoint, and demonstrated statistical superiority Non-inferiority established as the lower limit of the 95% confidence interval around the treatment difference was above -10 Superiority established as the lower limit of the 95% confidence interval around the treatment difference was above 0 (1) (1) adhoc p value = 0.020 Note: Due to insufficient sample size in the resistant population, no conclusions can be drawn for that population
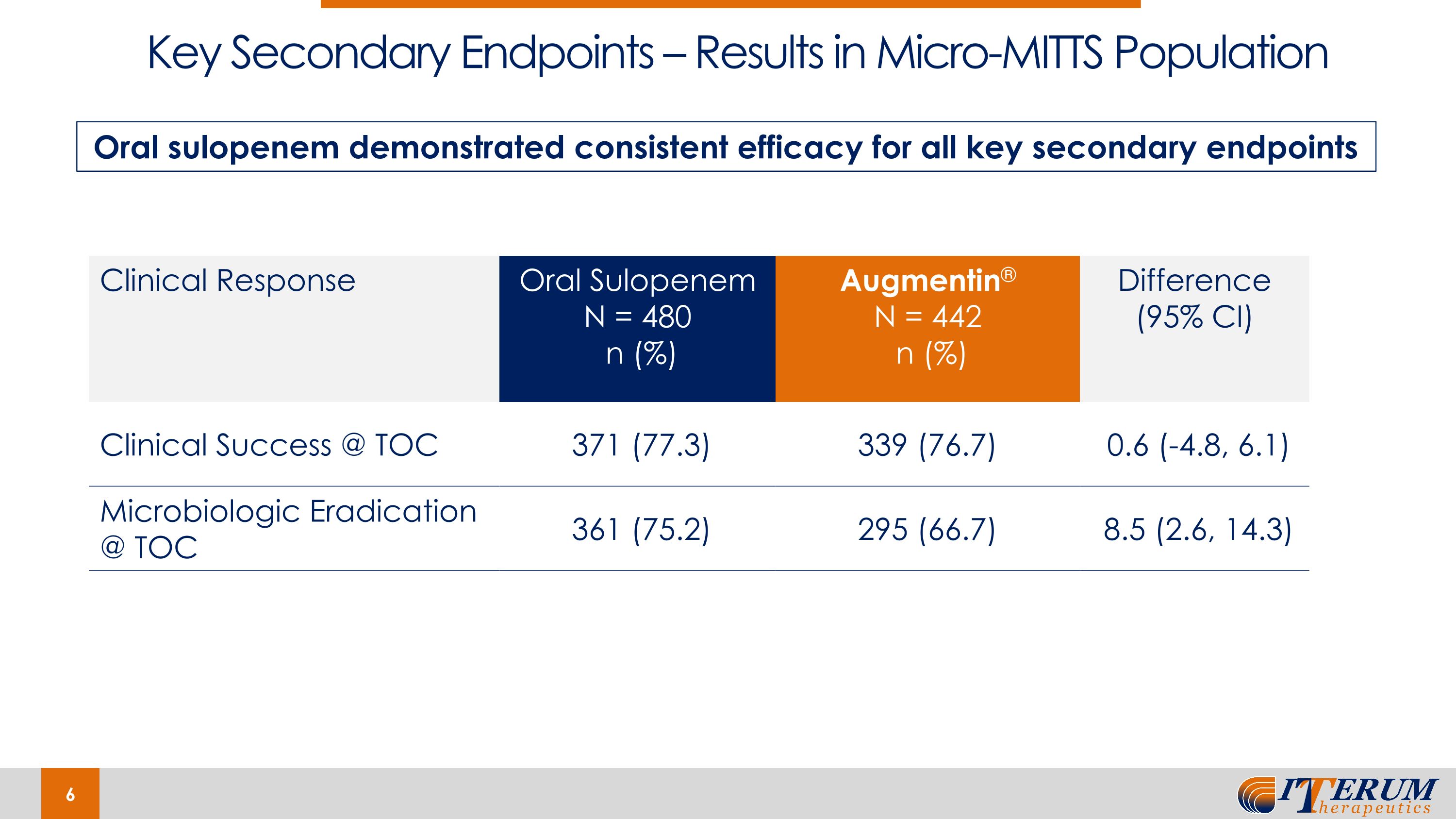
Key Secondary Endpoints – Results in Micro-MITTS Population Clinical Response Oral Sulopenem N = 480 n (%) Augmentin® N = 442 n (%) Difference (95% CI) Clinical Success @ TOC 371 (77.3) 339 (76.7) 0.6 (-4.8, 6.1) Microbiologic Eradication @ TOC 361 (75.2) 295 (66.7) 8.5 (2.6, 14.3) Oral sulopenem demonstrated consistent efficacy for all key secondary endpoints
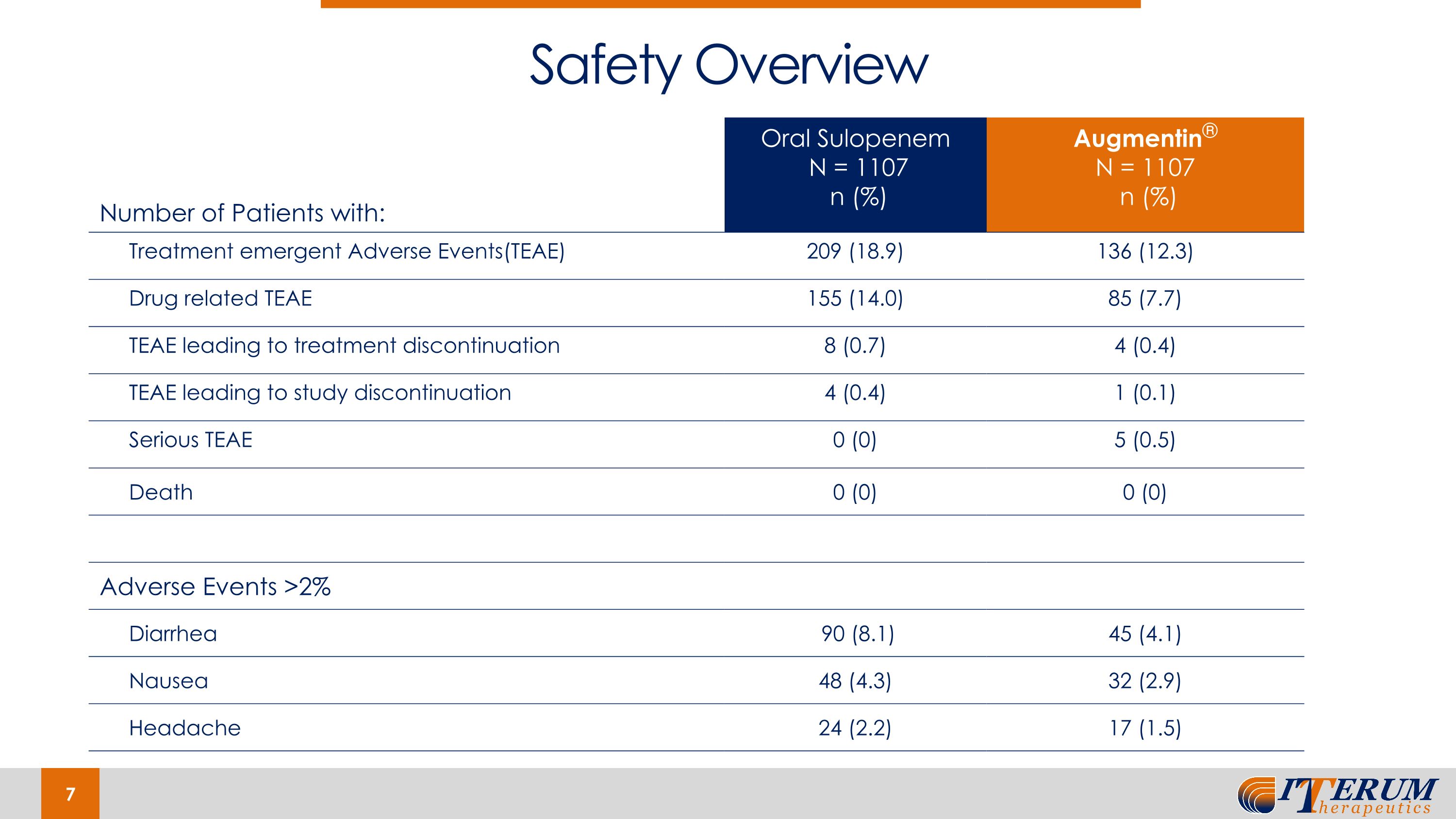
Number of Patients with: Oral Sulopenem N = 1107 n (%) Augmentin® N = 1107 n (%) Treatment emergent Adverse Events(TEAE) 209 (18.9) 136 (12.3) Drug related TEAE 155 (14.0) 85 (7.7) TEAE leading to treatment discontinuation 8 (0.7) 4 (0.4) TEAE leading to study discontinuation 4 (0.4) 1 (0.1) Serious TEAE 0 (0) 5 (0.5) Death 0 (0) 0 (0) Adverse Events >2% Diarrhea 90 (8.1) 45 (4.1) Nausea 48 (4.3) 32 (2.9) Headache 24 (2.2) 17 (1.5) Safety Overview

REASSURE Study Results In the overall response at the test of cure in the Augmentin susceptible population, oral sulopenem was non-inferior to Augmentin®, thereby achieving the primary endpoint; in this population, sulopenem also demonstrated statistical superiority Additionally, oral sulopenem demonstrated consistent efficacy for key secondary/additional endpoints Very solid safety profile Timing/Next Steps Expect to resubmit NDA Q2 2024 Expect FDA to complete its review and take action within six months from resubmission or in Q4 2024* Market Dynamics The uUTI market is quite large, with an estimated 30 million infections annually Antibiotic resistance and the safety profiles of existing older products currently in the market are driving a substantial need for new, efficacious products to treat these infections If approved, sulopenem would be the first oral penem to be approved in the United States Additionally, if approved, sulopenem would be one of the first new oral products approved for uncomplicated urinary tract infections since the turn of the century With positive data now in hand, we will focus on a strategic process to sell, license, or otherwise dispose of our rights to sulopenem with the goal of maximizing value for our stakeholders Summary
